Allgemeine Kontaktinformationen:
Anschrift:
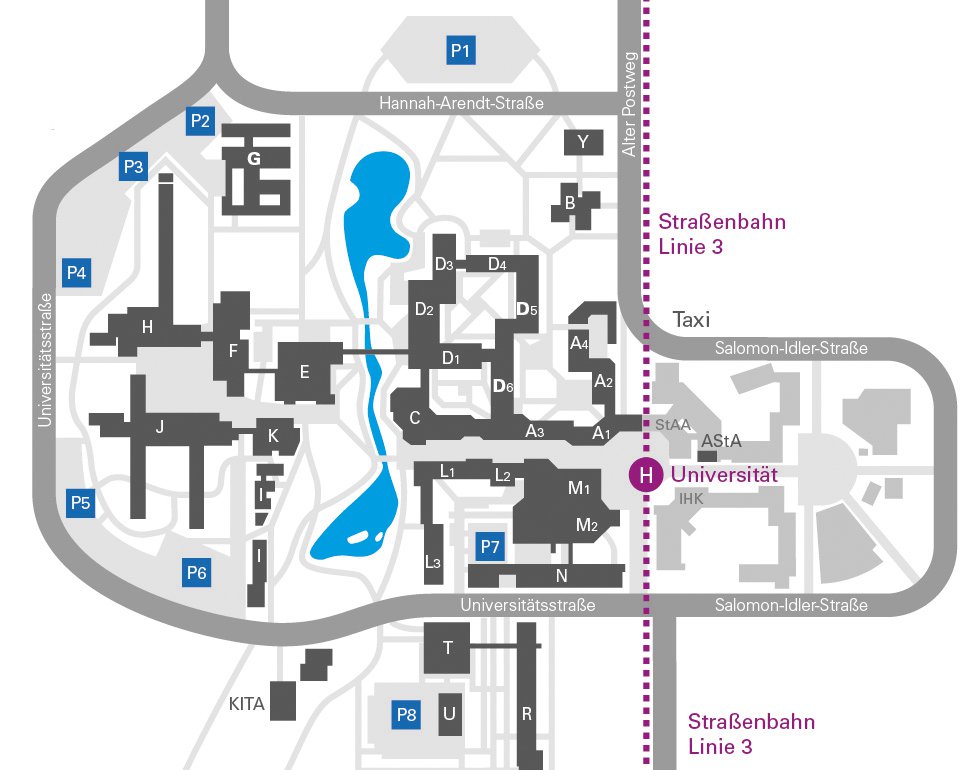
Universität Augsburg
Institut für Physik
Lehrstuhl für Festkörperchemie
Universitätsstr. 1
86159 Augsburg
Telefon: +49 821 598 -3032 (Sekretariat)
Fax: +49 821 598 -5955
E-Mail: sekretariat-fkch@physik.uni-augsburg.de (Sekretariat)
Gebäude: R (Sekretariat: Raum 436)